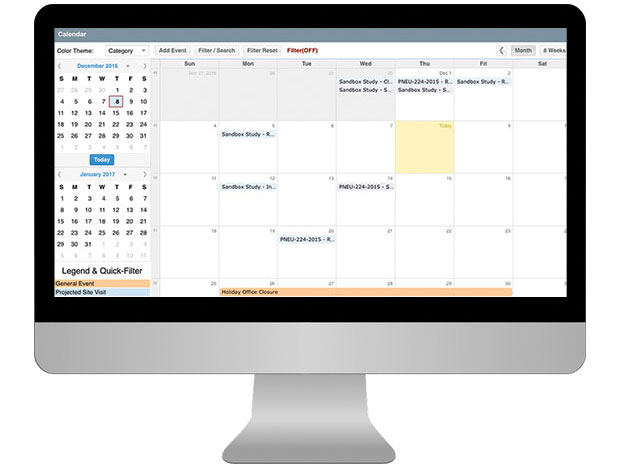
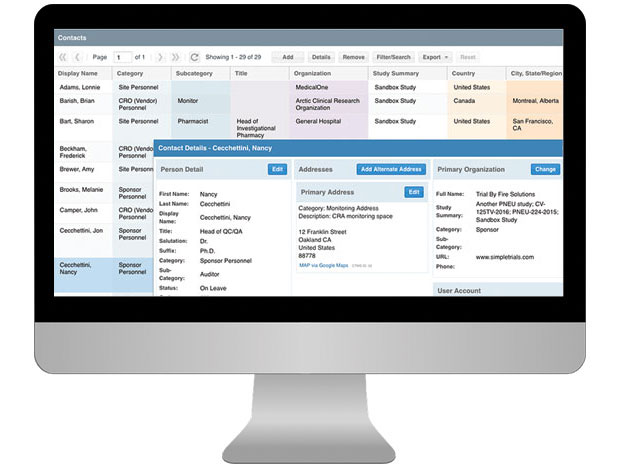
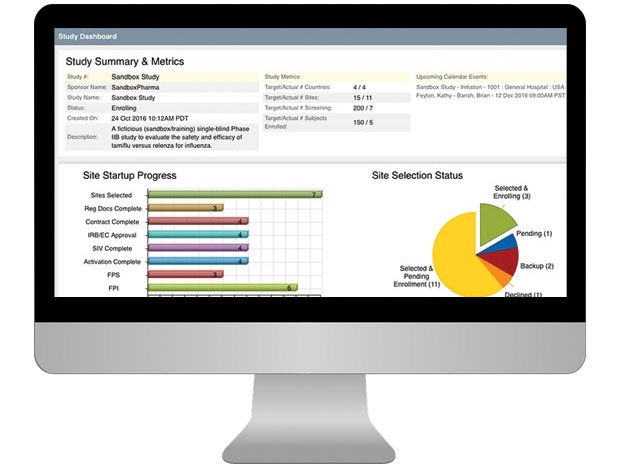
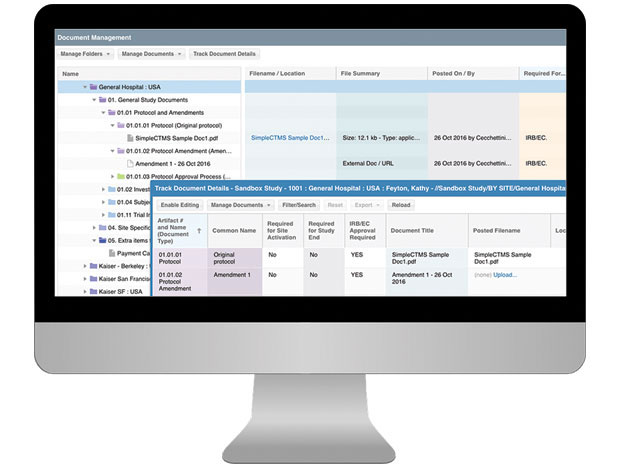
CTMS For Sponsors
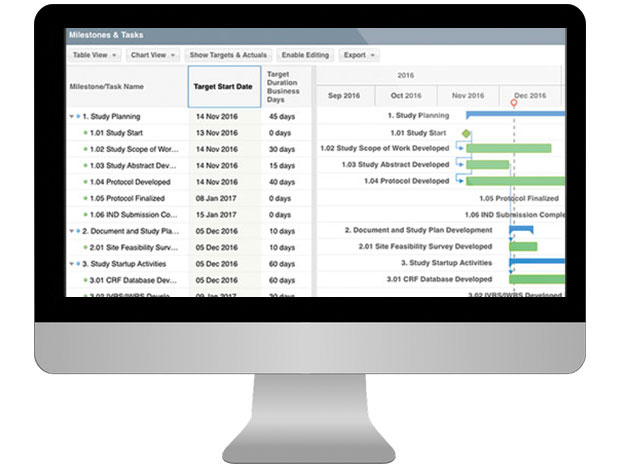
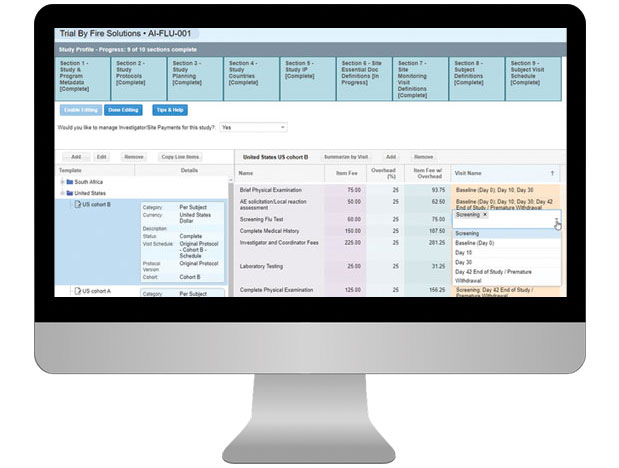
Having a well-organized CTMS that can easily support your outsourcing model, study pipeline and global team is critical to your success. SimpleTrials comes with just the right features, is scalable for your team growth, and comes with an affordable pricing model and no long term commitments.