What is a CTMS?
The Clinical Trial Management System is the essential set of tools to effectively plan, manage and track your clinical study portfolio. It is a specialized, comprehensive project management application that takes the study team from startup, through enrollment and monitoring, to study close.
Eight Features of a CTMS
The CTMS is a suite of eClinical tools that are most effective when they are all working together seamlessly:
Contact Management - Sites & Teams
Calendar & Monitoring
Document Management & eTMF
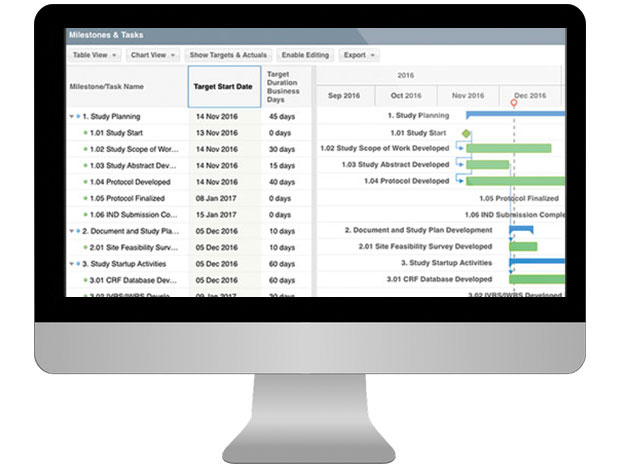
Project Plan - Study Milestones & Tasks
Contract & Payment System
Subject Tracking & EDC Integration
Visit Report Authoring & Letter Generation
Reporting and Business Analytics
The Benefits of CTMS
Access to accurate, up-to-date, study information
One of the challenges our team faced in our prior roles working directly for sponsors and CROs to manage clinical trials, was access to accurate up-to-date information about the trial. In some cases, it was difficult to get high level information like an updated site roster, and sometimes more granular such as startup tracking or site visit schedules. This is no way to run a study. Having a CTMS solution provides transparency and unified access to study information, so the study team can do their job and make sound decisions.Collaboration
The CTMS is a great place for members of a study team to work together, as well as supporting collaboration across teams (e.g. sponsor, CROs, sites). Team members can collaborate on a single task for the same study, such as study startup, with the confidence that they are all accessing the latest data. Sponsors, CROs, Sites and other vendors can also collaborate to share the responsibility of keeping study tracking data up to date.Efficiency
The CTMS is a specialized productivity tool that helps your busy study team to plan, track and monitor the study effectively. For example, an electronic visit report authoring feature can automatically integrate the details of your visit (study, site, date, investigator, monitor name, etc.) and automatically check that you have completed the required sections (Word can't do that). The Payment feature can automatically create site payment tracking records, based on your contracts, when subject visits are marked as completed.Oversight
The CTMS provides transparency to oversee the critical components of your study management, including study startup, screening & enrollment, document collection, site visits, monitoring reports, subject visit completion, action items and issue management, and more. Dashboards and data reports provide visualizations and performance scoring for one study or aggregated views of multiple studies.
Choosing the Right CTMS
Here are some of the common considerations when reviewing eclinical systems and making a selection of the right clinical trial management software to fit your needs:
Feature Set
Are there specific feature requirements or study management pain points that are a must-have for the system?Flexibility & Customization
Does the CTMS need to mold to your organizations' practices and policies in terms of field picklists, custom tracking fields and even custom built planning and tracking views (as an alternative to building out another spreadsheet tracker).Ease of Use
Do you need a system that your team can start using on day 1?Validation & Compliance
Do you plan to use the CTMS as the system of record for regulated data, such as site essential documents or electronic visit reports? If so, a validated system with controls for 21 part 11 will be critical.Support
Is it important that the study management system is supported by a help desk that understands clinical studies? Do you want the option of professional services and client management from your CTMS provider?Cost
Are you looking for a system with transparent pricing, which can start small and scale up? Is there an additional fee for adding studies to the CTMS? Is there a startup fee to get the workspace launched? Is a multi-year agreement required? Are there optional services you may need in the future, that you should consider now?
Compliance and CTMS
Here are some important considerations for Compliance with a CTMS, including support for 21 CFR Part 11:
Audit Trail
The CTMS should create a complete audit trail for all data changes made in the system, including who made the change, when, and the old and new values.Archival Policy
Sometimes it is appropriate to remove records from the CTMS; however, the CTMS should be archiving data rather than deleting the data. The main difference is that archived data can be easily restored, and deleted data cannot. Archival adds protection for unintended or malicious attempts to remove data from the system.Electronic Signatures
The CTMS should support electronic signatures for records that are relevant to the regulatory requirements of your study. For the CTMS, much of the planning and tracking is intended for the efficient and effective execution of the study, but may not be relevant to regulatory agencies (e.g. the study project plan with target vs. actual milestone dates). However, eTMF and document management as well as electronic visit reports are relevant, and should support electronic signatures.Account Controls
The CTMS should require that users be authorized and authenticated to the system, with controls around password expiration, idle session expiration, and locking of inactive accounts.
CTMS vs EDC
CTMS and EDC are complementary products, and most clinical studies use both. Electronic Data Capture (EDC) is focused on collecting patient data, and CTMS is focused on the project management aspects (which can include startup, documents, payments and monitoring) of the clinical trial. The systems do intersect, and it is beneficial to have a subset of the EDC data integrated to your CTMS. Those areas include:
High level screening, screen failure and enrollment data. The CTMS typically tracks anonymized subject records, so that the study management team can track enrollment at a high level, and also use that data for site payment tracking, visit report authoring, etc.
Subject visit progress, including actual visit dates and visit status.
Data collection status, including review, verification and/or approval of data.
End of study disposition (e.g. completed, discontinued) and date for each subject.
Excel vs CTMS?
Worksheet and spreadsheet trackers are a common tool for clinical study tracking. Worksheets are a quick and easy way to enter, view and manipulate data. They serve as a handy tool, but the limitations of worksheets for clinical trial management arise quickly as your team and study grows, and your organization seeks to lower risk through quality and compliance. Here are some of the advantages to using a CTMS over worksheets:
Collaboration with User Roles
The CTMS provides a secure location where team members can collaborate in a controlled environment, where users can be restricted to certain data view and/or certain studies.Integrated, Consistent Data
The CTMS provides a warehouse of data that can be applied consistently across data views within a study, and applied consistently across studies.Secure and Reliable
The CTMS provides controlled user accounts, so that data is accessed by authorized users only. High availability cloud computing technologies, backups and redundancy keep the data available when you need it.Compliant
The CTMS enforces controls for 21 CFR Part 11 compliance, seamlessly in the background as your team performs their study management activities. Controls include: audit trail, electronic signatures, data archival and user account controls.