Features
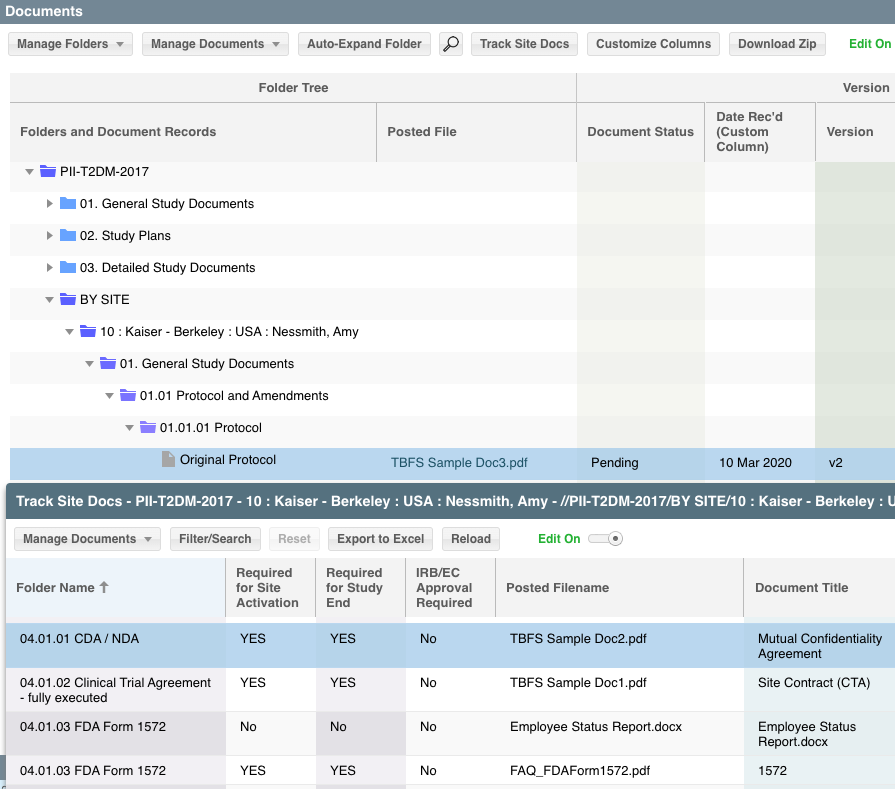
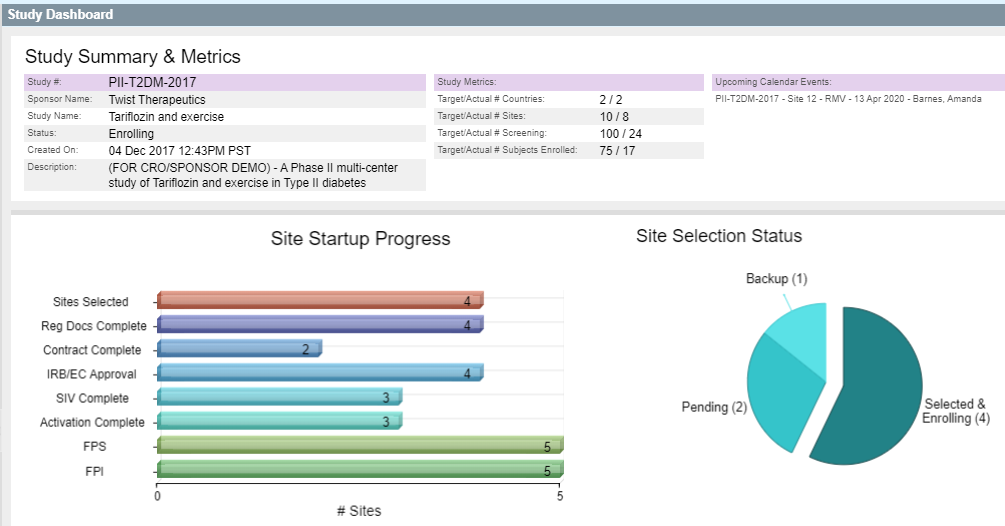
SimpleTrials’ versatile fully validated CTMS keeps everything you need — worksheets, documents, calendars, contacts, schedules, payments, milestones and more — in one feature-rich, scalable package all while maintaining compliance every step of the way. It’s the industry’s first on-demand, subscription-based CTMS for sites, vendors and sponsors.
Plans start at $599/month. With SimpleTrials, you subscribe, sign in and get to work with a comprehensive toolkit with no long-term commitments.